Select the Properties of the Sn2sn2 Reaction Mechanism.
Unimolecular at rate-determining step e. On the left we have an alkyl halide and we know that this bromine is a little bit more electronegative than this carbon so the bromine withdraws some electron density away from that carbon which makes this carbon a little bit positive so we say partially positive.
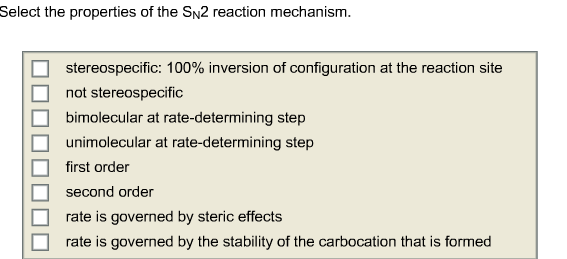
Select The Properties Of The Sn2 Reach Mechanism Chegg Com
Select the properties of the Sn1 reaction mechanism.
. Describing a step in a reaction mechanism in which only one particle undergoes a chemical change at the transition state. In this generic reaction the nucleophile is attacking the carbon holding the leaving group which causes the nucleophile to dissociate. Draw the product species to show the balanced equation including nonbonding electrons and formal charges.
Select the properties of the Sn2 reaction mechanism. Up to 256 cash back Write the mechanisms and products of each reaction. Nucleophilic substitution reactions are those reactions in which nucleophile attacks the alkyl halid.
Select the properties of the Sn2 reaction mechanism. It can be represented using the chemical formula P 20 5It has a molecular weight of 141945 grams per mole and its density is about 239 Gcm 3It can also be termed as Diphosphorus Pentoxide Phosphorus V Oxide Phosphoric Anhydride Tetraphosphorus Decaoxide Tetraphosphorus Decoxide. 8 fastest -iodide -bromide.
For alkyl halides used in SN1SN1 and SN2SN2 mechanisms rank the leaving groups in order of reaction rate. So the product assumes a stereochemical position opposite to the leaving group originally occupied. Give the major product of the following reactions and indicate the mechanism eg.
100 inversion of configuration at the reaction site b. It goes straight from reagents to products. 100 inversion of configuration at the reaction site rate is governed by steric effects rate is governed by the stability of the carbocation that is formed O not stereospecific second order.
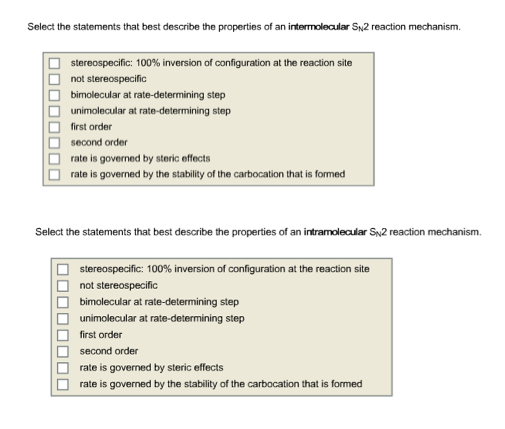
Select the statements that best describe the properties of an intermolecular S_N 2 reaction mechanism. Draw the structure of reactants and products on the diagramYou can put the reactants at any energy level and then draw the. 93 30 ratings Transcribed image text.
Question 5 The Energy Diagram of SN2 reaction. Oscillations Redox Reactions Limits and Derivatives Motion in a Plane Mechanical Properties of Fluids class 12 Atoms Chemical Kinetics Moving Charges and Magnetism Microbes in Human Welfare Semiconductor Electronics. The SN2 reaction mechanism is a bimolecular reaction mechanism.
- Instructor Lets look at the mechanism for an SN2 reaction. I If the substance is an R enantiomer then in the SN 2 reaction the nucleophile will attack the substrate from the front end. 285 Bought 3 Share With.
Add curved arrows to the reactant side of the following SN2 reaction to indicate the flow of electrons. SN2 SN2 CH3 CH3 E2. Select the properties of the SN2reaction mechanism bimolecular at rate-determining step first order unimolecular at rate-determining step stereospecific.
S N 2 reaction mechanism requires the attack of nucleophile from the back side of the carbon atom. 100 inversion of configuration at the reaction site not stereospecific bimolecular at rate-determining step unimolecuiar at rate-determining step first order second order rate is governed by steric effects rate is governed by. So It is bimolecular at the rate-determining step.
- It is Single step reaction. Bimolecular at rate-determining step d. Rate of reaction K R L Nu It is a second order reaction.
Select the properties of the SN2SN2 reaction mechanism A. E1 through which. Notice that the net charge of the reaction stays the same.
The SN2 reaction is a good example of stereospecific reaction one in which different stereoisomers. Do not draw out the mechanism KOCCH33 in CH33COH b OTs c Br Br CH3CH2CH2OH warm d CH 3 CH2CH3 H OTs KCN in acetone 20oC e Br f I CH3. Select the properties of the SN2SN2 reaction mechanism A.
SN2 reaction was supposed to be n-butyl bromide primary so that mechanism would be helpful also. 100 inversion of configuration at the reaction site not stereospecific bimolecular at rate-determining step unimolecular at rate-determining step first order second order rate is governed by steric effects. The SN2 mechanism is through the backside attack.
Different properties of SN² reactions. SN 2 reactions are stereospecific and can sometimes lead to a change in the stereochemical properties of the end product. Transition state is formed which is.
Theres no intermediate in this reaction. Select the properties of the SN2 reaction mechanism. This does not cause any change in stereochemistry and the.
This is called inversion of configuration. Rate is dependent on concentration and strength of Nucleophile. First order second order rate is governed by steric effects frate is governed by the stability of the carbocation that is formed.
This is because SN 2 reactions can occur primarily in two ways. Also state the mechanism through which each reaction proceeds eg. Rank the SN2 reaction rate of the following species.
Draw an energy diagram for the following S N 2 reaction. Materials Devices and Simple Circuits. Select the properties of the Sn2 reaction mechanism.
Label the axes the Ea the ΔH and the transition state of the reactionAssume the reaction is exothermic and ΔH -75 kJmol and Ea 50 kJmol. Nucleophilic substitution reactions are those reactions in which nucleophile attacks the alkyl halid.
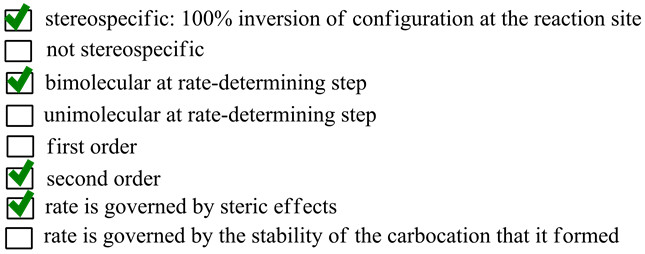
Select The Properties Of The Sn2 Reaction Mechanism Stereospecific 100 Inversion Of Configuration Home Work Help Learn Cbse Forum
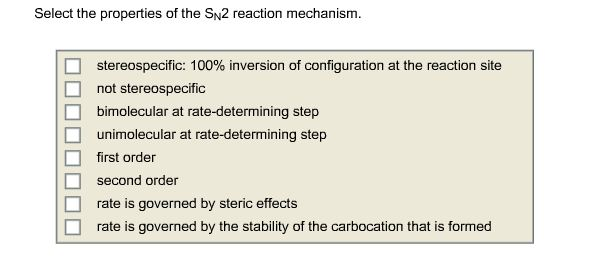
Solved Select The Properties Of The Sn2 Reaction Mechanism Chegg Com

Solved Select The Statements That Best Describe The Chegg Com

Comments
Post a Comment